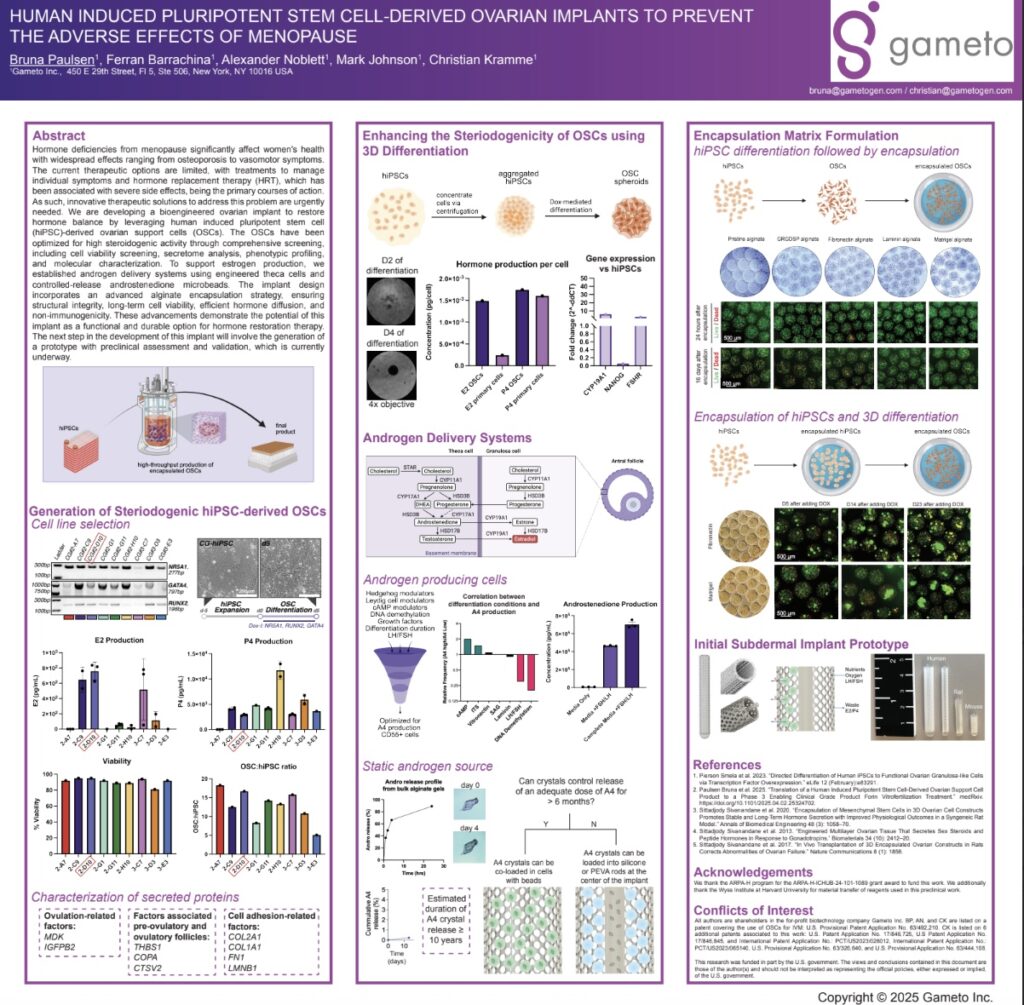
Background: Hormone deficiencies from menopause significantly affect women’s health with widespread effects ranging from osteoporosis to vasomotor symptoms. The current therapeutic options are limited, with treatments to manage individual symptoms and hormone replacement therapy (HRT), which has been associated with severe side effects, being the primary courses of action. As such, innovative therapeutic solutions to address this problem are urgently needed.
Objective: We are developing a bioengineered ovarian implant to restore hormone balance by leveraging human induced pluripotent stem cell (hiPSC)-derived ovarian support cells (OSCs).
Methods: The OSCs have been optimized for high steroidogenic activity through comprehensive screening, including cell viability screening, secretome analysis, phenotypic profiling, and molecular characterization. To support estrogen production, we established androgen delivery systems using engineered theca cells and controlled-release androstenedione microbeads. The implant design incorporates an advanced alginate encapsulation strategy, ensuring structural integrity, long-term cell viability, efficient hormone diffusion, and non-immunogenicity.
Results and Next Steps: These advancements demonstrate the potential of this implant as a functional and durable option for hormone restoration therapy. The next step in the development of this implant will involve the generation of a prototype with preclinical assessment and validation, which is currently underway.