Objective: Historically, human oocytes from in vitro maturation (IVM) have suffered from poor developmental competence, likely owing to insufficient cytoplasmic maturation. We recently described a method to generate ovarian support cells (OSCs) through directed differentiation of human induced pluripotent stem cells (hiPSCs). Here we compare the oocyte quality of oocytes from OSC-IVM, traditional media IVM, and in vivo maturation using single oocyte RNA sequencing.
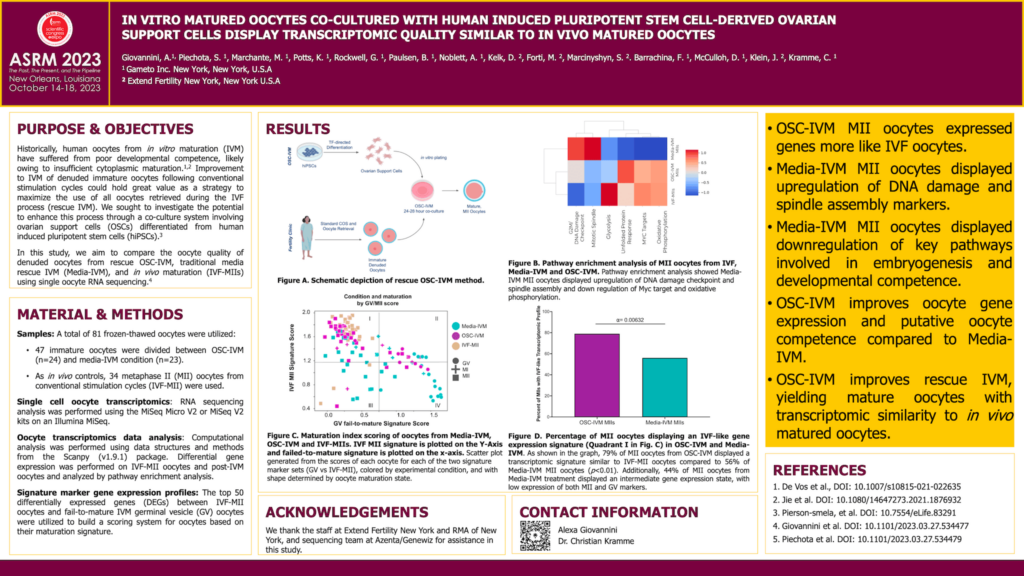
Materials & Methods: A total of 81 oocytes were utilized for RNA-seq analysis: 34 metaphase II (MII) oocytes obtained from conventional gonadotropin stimulation (IVF-MII), 24 IVM treated oocytes from OSC-IVM, and 23 oocytes from a media-IVM condition. Individual oocytes were harvested for RNA-sequencing library preparation using NEBNext Ultra Low Input RNA-Seq library prep kit and sequenced on an Illumina MiSeq. Raw reads were processed via STAR and gene counts were normalized and analyzed using scanpy. Differential gene expression was performed between the IVF-MII oocytes and the post-IVM germinal vesicle (GV) oocytes and the top 50 differentially expressed genes (DEGs) were utilized to build a scoring system for oocytes based on their maturation signature.Data was plotted using Uniform Manifold Approximation and Projection(UMAP) and leiden clustering was used to characterize whole transcriptome similarity.
Results: We identified three clusters (0, 2, and 3) within the MII oocytes population, and one cluster (1) comprised mostly GVs. As expected, the GV maturation signature was strongly represented in cluster 1. Similarly, the MII maturation signature included MIIs from both IVF and IVM, and IVF MIIs were more overrepresented in clusters 0 and 2 compared to cluster 3. As expected, the strong majority (91%) of MII oocytes from the IVF-MII condition were classified within clusters 0 and 2. As a continued indication of positive maturation impact, co-culture with OSCs led to generation of 79% MII oocytes with a ‘IVF-like MII’ profile (cluster 0 and 2). In comparison, just 56% of resultant MII oocytes from the Media-IVM condition were found in ‘IVF-like MII’ profile clusters. This population distribution is significantly different from random (c2 test, a = 0.00632). To assess for confounding variables in our transcriptomic analysis, we evaluated expression of cell cycle, apoptosis and oxidative stress genes and did not detect any significant patterns, indicating that the oocytes do not exhibit major signs of stress or breakdown.
Conclusions: Oocytes matured in the OSC-IVM condition shared broad transcriptomic similarity with in vivo matured oocytes compared to the Media-IVM oocytes and displayed no major signature of oxidative stressor degradation.
Impact Statement: The use of ovarian support cells (OSCs) derived from human induced pluripotent stem cell (hiPSCs) in a co-culture system isa novel and beneficial approach to improve oocyte in vitro maturation and promote more in vivo-like oocyte gene expression profiles.