Fertilo: The Future of IVF and Egg Freezing
Gameto is on a mission to study ways to improve the IVF and egg freezing process by reducing the burden on women and families.
Fertilo Has Been Designed to Enable Rapid Egg Maturation Outside the Body
Fertilo is a solution aimed at reducing the number of hormonal injections needed for IVF and egg freezing.
It is designed to allow for much of the egg maturation process to happen outside the body (ex vivo), with the goal to achieve a shorter, easier, and more accessible cycle. The Fertilo cycle is designed to require a minimal hormonal stimulation protocol, physicians intentionally retrieve immature eggs, and Fertilo matures those eggs in the dish (in vitro).
The concept of maturing eggs outside the body, called in vitro maturation (IVM), is not new. As of 2021, the American Society for Reproductive Medicine (ASRM) no longer considers IVM to be an experimental procedure, and thousands of babies have been born by IVM since 1991. Fertilo is designed to make IVM more effective and, therefore, may be an attractive treatment option for many women and families seeking IVF and egg freezing.
How is Fertilo designed to work?
1
Shortened Hormonal Stimulation
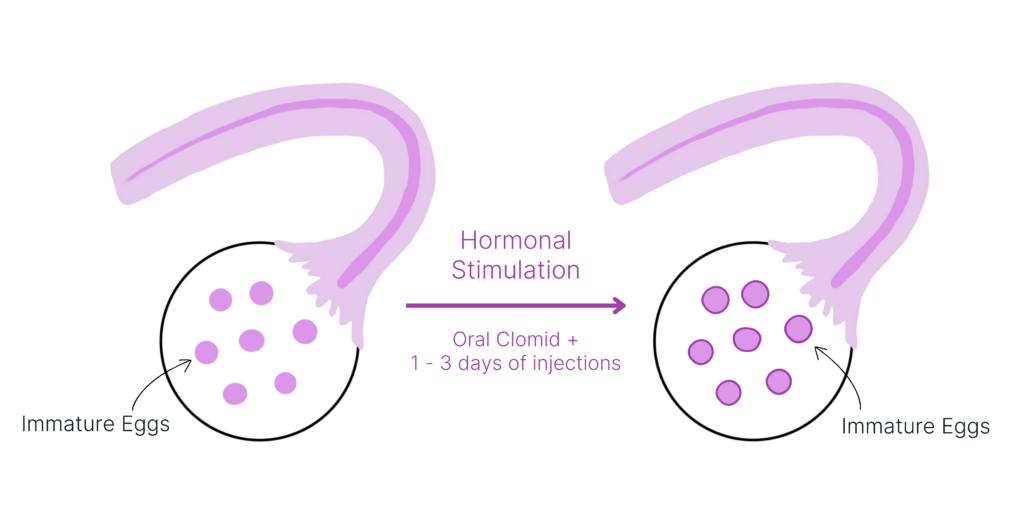
Fertilo is designed to require just 1 to 3 days of hormonal stimulation to prepare your immature eggs for collection. Fewer hormones means fewer side effects, less time spent at the clinic, and an easier cycle overall.
2
Immature Egg Retrieval
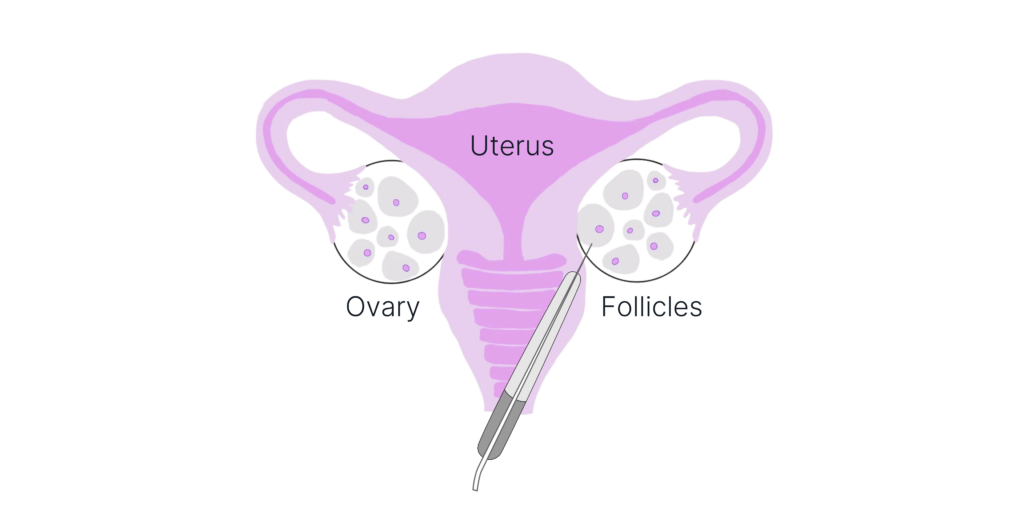
Immature eggs can be retrieved during a short outpatient procedure at a fertility clinic, just like a conventional cycle.
3
Egg Maturation with Fertilo
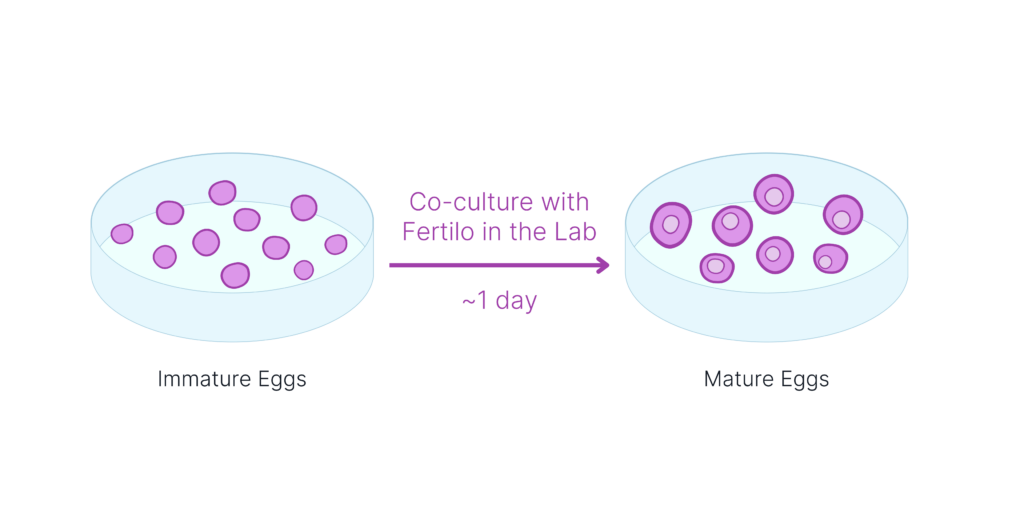
Immature eggs are placed in a dish with Fertilo for ~1 day. Fertilo is designed to mimic a young ovarian environment, and to replicate the body’s natural maturation process by responding to the eggs’ needs and producing necessary hormones and nutrients.
4
Fertilization or Freezing
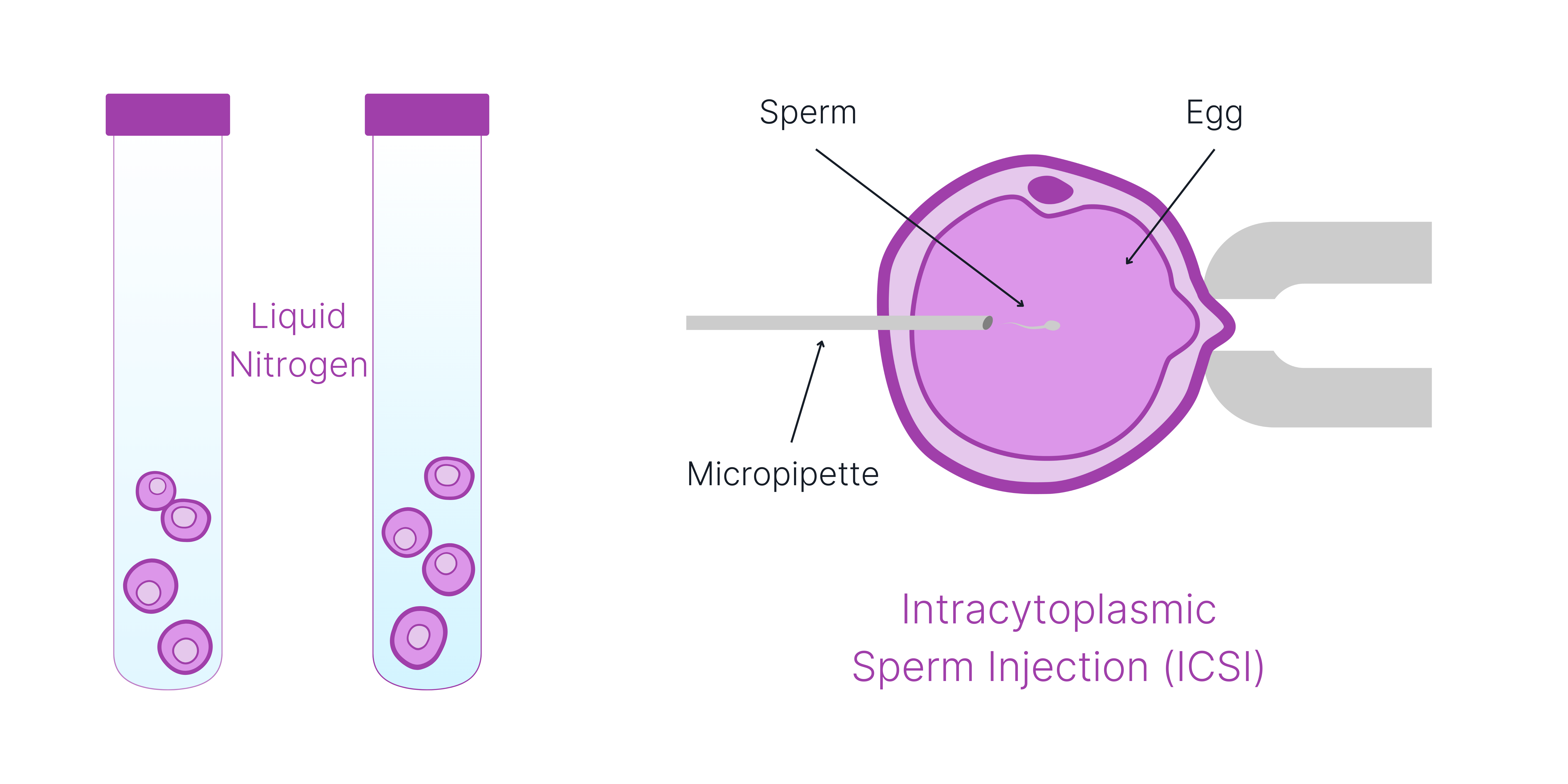
The rest of the Fertilo cycle looks exactly the same as a conventional IVF or egg freezing cycle. The eggs will either be fertilized or frozen for future use.
Fertilo is designed to offer an alternative to full stimulation for those who need it – or want it.

“I may not respond well to stimulation.”
Maybe you’ve been diagnosed with PCOS. Or perhaps your body just doesn’t respond normally to stimulation.
“I may not actually need full stimulation.”
Maybe your partner is infertile. Perhaps you’re in a same sex partnership. Or maybe you’re just freezing your eggs for the future.


“I am just trying to avoid all the injections.”
Maybe you need fertility treatment but are anxious about the injections. Or perhaps you just want to try something different.
See how Fertilo could help you along your fertility journey.
80% Reduction in Overall Symptoms
In a study conducted across the United States and Spain, participants who underwent the Fertilo protocol reported:
No Breast Swelling
86%
No Pelvic / Abdominal Pain
76%
Regular Menstrual Cycle Month Post Retrieval
86%
No Bleeding / Fever Post Retrieval
98%
No Nausea / Vomiting
96%
No Severe Pain
92%
Among study participants who underwent both a Fertilo and conventional cycle, 92% of patients would repeat the Fertilo cycle, while only 77% would repeat the conventional cycle
Study participants reported an 80% reduction in overall symptoms among patients who have undergone both cycle types
A significant difference was observed in the severe pain levels experienced by participants following the conventional and minimal stimulation cycles, with participants reporting 38% and 8%, respectively
Please note that prior study results may not be reflective of the safety and effectiveness of Fertilo
Source: Donor side effects experienced under minimal controlled ovarian stimulation with in vitro maturation vs. conventional controlled ovarian stimulation for in vitro fertilization treatment
Where is Fertilo available?
Fertilo is currently being investigated in a Phase 3 clinical trial and is not approved for marketing in the United States. Fertilo will be evaluated for safety and effectiveness as part of this Phase 3 study. Fertilo is currently available in Australia, Argentina, Peru, and Mexico.
Interested in Fertilo?
Sign up below to receive updates on Fertilo, including availability in your area (outside the U.S. only).